Funder:
Principal Investigator(s):
Location(s):
Hospital for Tropical Diseases, HCMC, Vietnam
Dengue incidence continues to rise globally, with an estimated 3.9 billion people at risk each year. During the rainy season, patient consultations and hospital admissions often surge, overwhelming healthcare systems, particularly in tropical and subtropical regions where resources and clinical capacity are limited.
As there are currently no effective antiviral or therapeutic agents, dengue management depends largely on close monitoring and timely supportive care. Frequent measurement of vital signs and serial haematocrits remains central to diagnosis and prognosis, especially in low- and middle-income countries (LMICs) where continuous monitoring devices are rarely available. Conventional tools such as continuous blood pressure monitors or cardiac output devices are often prohibitively expensive, require specialist training, and are impractical in resource-limited settings.
To address this critical gap, we are collaborating with Imperial College London to develop D-SCAPE (Dengue Shock Classification And Prediction wEarable), a novel, affordable, and non-invasive wearable device designed for continuous real-time monitoring of physiological parameters relevant to dengue pathophysiology. By enabling early identification of severe progression, the device aims to support timely intervention, optimise fluid management, and ultimately improve outcomes for patients in resource-limited settings.
As part of the Wellcome Trust–funded Innovations Flagship Programme, engineers and researchers at Imperial College London and our research team have developed machine learning algorithms capable of classifying dengue severity and predicting severe outcomes within the next six hours using only photoplethysmography (PPG) data. Building on this foundation, D-SCAPE uses multi-wavelength PPG sensors to derive pulse rate, oxygen saturation, haematocrit, blood pressure, and other key physiological indicators.
D-SCAPE Prototype Development
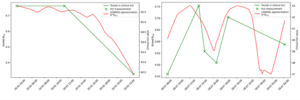
In 2022–2023, we tested the first prototype, D-SCAPE v1, on both healthy volunteers and dengue patients with varying severity levels. Results showed that the device reliably differentiated between severe and non-severe dengue cases. PPG-based estimates of SpO₂ and heart rate demonstrated high accuracy (mean absolute errors of 1.54% and 4.1 bpm, respectively), meeting commercial and FDA standards.
Importantly, D-SCAPE v1 also tracked haematocrit changes closely aligned with laboratory results, revealing trends that conventional blood tests might miss. Patient feedback was overwhelmingly positive—most participants expressed willingness to wear the device if it could lead to faster discharge, while recommending further miniaturisation for comfort.
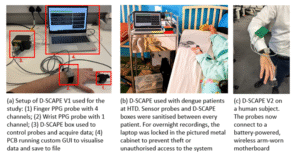
Advancing to D-SCAPE v2 and v3
Building on our earlier findings, we have developed the next versions of D-SCAPE prototypes. This device is smaller, fully battery-powered, and capable of wireless data transmission, offering greater patient comfort and freedom of movement. It also enables precise signal synchronisation for accurate non-invasive blood pressure estimation, representing a major step forward in continuous, real-time dengue monitoring. The project will move through a two-stage clinical development pathway to refine and validate D-SCAPE further.
Aims/objectives: