Tuberculous meningitis (TBM) is the most severe and life-threatening form of tuberculosis. Even with treatment, it carries a high fatality rate of 20% to 50%, and half of the survivors live with permanent neurological complications.
Most TBM research to date has focused on blood samples for sequencing and cell functions, while studies using cerebrospinal fluid (CSF) – the fluid surrounding the brain and spinal cord – remain rare. This is largely due to technical challenges, including the invasive nature of CSF collection, low cell yields, and the risk of blood contamination. However, to develop better treatments, researchers need a clearer picture of what happens at the actual site of infection – the brain.
At OUCRU, a team of scientists applied single-cell RNA sequencing (scRNA-seq), an advanced technology, to study how immune cells behave in CSF. To our knowledge, this is the first time scRNA-seq has been applied to examine CSF in adult TBM patients in the world.
ScRNA-seq has transformed biomedical research globally. It allows researchers to analyse thousands of single immune cells at once, revealing a far more detailed picture of the immune response. Importantly, it can work with only a few thousands of cells, helping to overcome one of the key challenges in TBM research: low cell yields from CSF samples. This makes it possible to identify specific immune cells in the brain that drive inflammation, potentially improving TBM diagnosis and guiding targeted treatments to reduce immune-mediated brain injury.
In this pilot study recently published in The Journal of Immunology, OUCRU researchers analysed paired pre-treatment CSF and blood samples from four adults with TBM, and blood samples from three healthy controls. They successfully identified 15 major cell types and 29 subtypes present across both compartments, but significantly different in abundance and function.
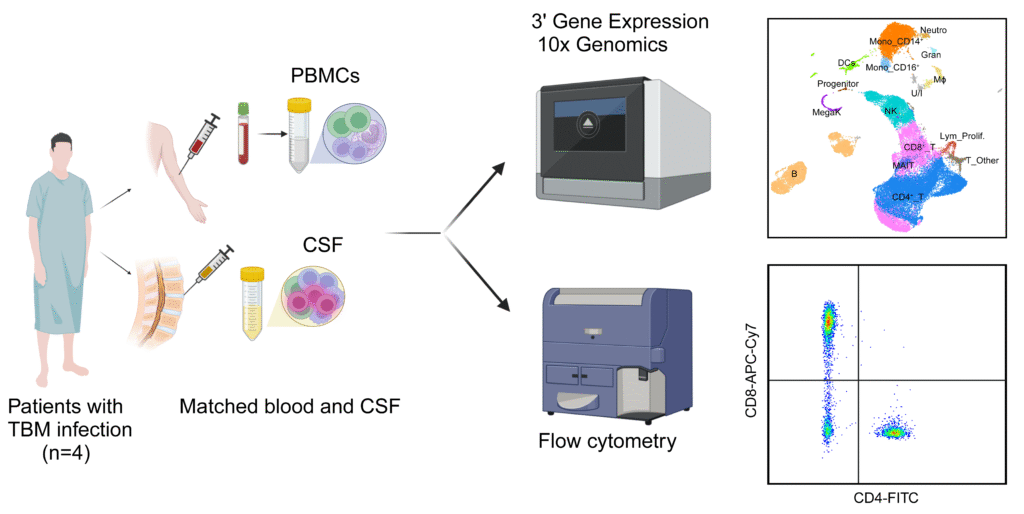
The CSF was enriched with immune cells that showed signs of inflammation, including microglia-like macrophages, GZMK+ CD8+ effector-memory T cells, and CD56bright NK (natural killer) cells. In contrast, the blood contained more cytotoxic GNLY+ CD8+ effector-memory T cells and CD56dim NK. Inflammatory signalling pathways, including interferon response, were more active in CSF cells, while oxidative phosphorylation, a key oxygen-based energy pathway, was reduced.
This metabolic shift reflects earlier evidence that, in TBM, immune cells switch from oxygen-based energy production to a faster oxygen-independent, lactate-producing glycolysis, similar to the ‘Warburg effect’ in cancer. The researchers hypothesise, though not yet confirmed, that this rapid-energy strategy may help fuel the immune response but also drive damaging inflammation in the brain.
By creating a detailed reference map of immune cell states in CSF and blood of adults with TBM, this pilot study offers an early and important resource for the TBM research community. It also confirms that scRNA-seq is feasible and highly informative for studying TBM immunopathogenesis, even with technically challenging sample types like CSF.
In establishing a clear workflow and analysis pipeline for paired CSF and blood, the team also laid a foundation for larger studies to explore disease mechanisms and develop new treatment strategies for TBM and other brain infections.
Dr Trịnh Thị Bích Trâm, first author, said: ‘Although scRNA-seq has been used internationally for nearly a decade, we only established it at OUCRU Vietnam two years ago, with strong support from the University of Oxford. This pilot was technically demanding, from the difficulty of obtaining fresh, high-quality CSF samples with enough viable immune cells to the complexity of new analytical approaches. Completing it is a major step forward for TB research in Vietnam, and I’m incredibly proud of OUCRU Tuberculosis Research Group and what we’ve achieved together.’
The full article Single-cell profiling of blood and cerebrospinal fluid in tuberculous meningitis is published in The Journal of Immunology.