- The VIETNARMS trial found that the readily available and affordable Hepatitis C treatment sofosbuvir/daclatasvir (SOF/DCV) had a cure rate of more than 95%.
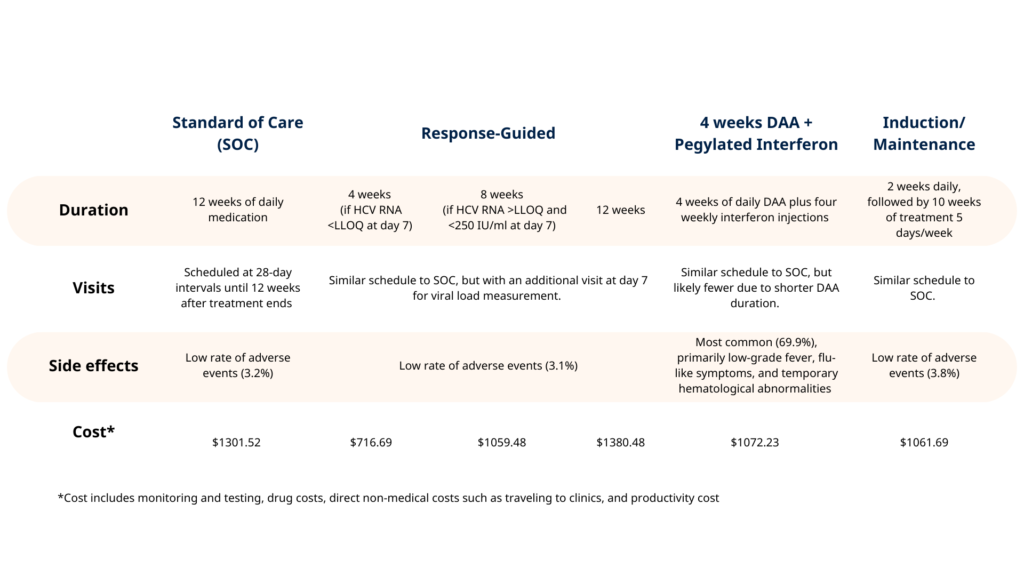
- The trial successfully tested several treatment strategies, including a response-guided, shortened treatment duration strategy that maintained effectiveness at half the cost compared to the standard 12-week, $1,300 treatment.
Hepatitis C is a global health concern, with an estimated 58 million people chronically infected. The World Health Organisation (WHO) aims to eliminate viral hepatitis as a public health threat by 2030, with effective treatment being crucial to this strategy. However, while several highly effective direct-acting antiviral (DAA) medications are available, they remain unavailable and expensive, especially in low- and middle-income (LMIC) settings such as Vietnam.
Currently, the standard course of treatment for Hepatitis C lasts 12 weeks and costs $1,300 (about 33 million VND). In Vietnam, only 50% of the treatment cost is covered by social insurance. The prolonged treatment duration and high cost can discourage patients from seeking or following up with treatment.
The VIETNARMS trial, which ran from 2018 to 2024 and enrolled 624 patients, directly compared two widely used hepatitis C treatment regimens: sofosbuvir/daclatasvir (SOF/DCV) and sofosbuvir/velpatasvir (SOF/VEL). This head-to-head comparison, the first of its kind, revealed that SOF/DCV demonstrated slightly higher cure rates compared to SOF/VEL. This finding is particularly significant for LMIC countries where SOF/DCV is more readily accessible and affordable.
Beyond comparing existing treatments, the VIETNARMS trial explored innovative treatment strategies designed to enhance accessibility and affordability. The research team investigated shorter treatment durations and a response-guided approach where treatment duration is tailored to individual patient response.
The results were promising. One strategy involving a response-guided approach demonstrated comparable effectiveness to the standard 12-week treatment duration while shortening the treatment duration and halving the treatment cost. These findings provide new strategies for expanding access to treatment, particularly for vulnerable and underserved communities, and have the potential to shape treatment guidelines worldwide.
This complex trial was made possible thanks to the multi-national collaboration between Imperial College London, the University of Oxford, OUCRU, as well as local partners in Vietnam including the Hospital for Tropical Diseases (Ho Chi Minh City), the National Hospital for Tropical Diseases (Ha Noi), CDC Ha Nam, CDC Ha Noi, regional hospitals, and community-based organisations.
Professor Graham Cooke, Principal Investigator of the trial, emphasised the importance of these findings: “Despite some previous theoretical concerns, these data support the use in Vietnam of SOF/DCV, the cheapest option for HCV treatment globally. There is the potential for a significant decrease in prices in the country which should help expand access and accelerate elimination of this major challenge to public health.”
Professor Dr Nguyen Van Vinh Chau, Deputy Director of Ho Chi Minh City Department of Health, said: “These research findings provide a solid basis for the WHO and Vietnam’s Ministry of Health to update and improve Hepatitis C treatment guidelines. It gives us a chance to shorten treatment time and use a combination of effective medicines at a more affordable cost.”
The VIETNARMS trial, with its rigorous design and comprehensive evaluation of both established and novel treatment approaches, represents a significant step forward in the search for treatments for hepatitis C. The study’s findings have the potential to shape treatment guidelines, inform healthcare policies, and ultimately improve the lives of millions affected by this disease globally.
To learn more about the results, visit the publication here.